Emergency recall! Xiaolin pharmaceutical health care products have caused 2 deaths and 106 hospitalizations. Attention should also be paid to these products →
According to CCTV news, according to a report by the Japan Broadcasting Association on March 27th, local time, the Japanese Ministry of Health, Labor and Welfare announced the second death case suspected of taking a health product containing monascus from Kobayashi Pharmaceutical Company on March 26th, and the number of people admitted to the hospital has increased to 106.
Earlier, the Japanese Ministry of Health, Labor and Welfare had investigated Kobayashi Pharmaceutical Company and found that there was a new death report in addition to a previously announced death case.
Based on the Food Hygiene Law, Japan’s Ministry of Health, Labor and Welfare has asked Osaka headquarters where Kobayashi Pharmaceutical Company is located to take measures including product abandonment.
In view of the fact that many people were hospitalized and two people died after taking products containing monascus from Kobayashi Pharmaceutical Company, the Japanese Ministry of Health, Labor and Welfare will hold a meeting with the Consumer Department, the Ministry of Agriculture, Forestry and Fisheries and other relevant departments on the 27th to discuss future countermeasures.
According to Japanese media reports on the 27th, the Osaka municipal government of Japan ordered Kobayashi Pharmaceutical Company to recall three products, including "red yeast cholesterol granules".


Consumers die after taking health care products.
Kobayashi Pharmaceutical Emergency Recall
Previously, Kobayashi Pharmaceutical Co., Ltd. said that a consumer who took the company’s health care products containing monascus ingredients died of kidney disease. Some consumers suffer from diseases in kidney and other parts after using this health care product, and they are hospitalized, and some even need dialysis.
On March 26, Kobayashi Pharmaceutical updated the notice asking consumers to stop using the company’s products containing monascus ingredients and to recall them independently. According to the notice, the consumer took the company’s health care product containing monascus ingredients-monascus cholesterol granules. However, the notice did not disclose the relevant information of the consumer and the time of death.

The notice said that it has been confirmed that the consumer continued to buy red yeast cholesterol granules from April 2021 to February 2024. The company is investigating whether there is a causal relationship between the consumer’s death and taking the above health care products.
According to Kyodo News, since February 2021, monascus cholesterol granules have been sold in e-commerce channels and stores, and about 1.06 million bags have been sold by the end of February this year.
Three products involved
Are health care products for improving cholesterol.
The following is a list of three recalled products released by Kobayashi Pharmaceutical Company official website, all of which are health products for improving cholesterol. Please be careful not to buy or continue taking it.

China e-commerce platform removes products involved.
Consumers can return goods and get a refund.
Kobayashi Pharmaceutical Co., Ltd. is an old Japanese pharmaceutical company with a history of more than 100 years, and many of its products are quite popular. According to the company, monascus is a raw material produced by mixing monascus with steamed rice for fermentation. The company publicizes that this ingredient is expected to reduce the cholesterol value of low density lipoprotein.
The reporter searched a number of domestic e-commerce platforms and found that there are currently no three related products sold on the official e-commerce platform of Xiaolin Pharmaceutical, but there are still purchasing merchants selling related recalled products.
Kobayashi Pharmaceutical China Company said, "The company has received reports that it may cause kidney diseases and other problems after eating, and some of its products are sold to China through cross-border channels. If a consumer has eaten it and feels unwell, contact customer service, and the products that have not been eaten will be returned and refunded. "

On March 22nd, @ Kobayashi Pharmaceuticals issued a notice to remind consumers who have red herring cholesterol granules (60, barcode 4987072059708) to stop eating.
Up to now, the products involved have been removed from the official store of Kobayashi Pharmaceutical, the e-commerce platform mentioned in the notice. According to the reporter’s inquiry, multiple e-commerce platforms have been unable to search for this product.

Kobayashi pharmaceutical president bowed and apologized.
Admit that decision-making is slow
On the evening of March 22nd, President Kobayashi held a press conference and bowed to apologize for this issue.

According to Japanese media reports, the analysis results on the 16th of this month showed that some raw materials of monascus may contain unknown ingredients, but the company did not announce the recall until the 22nd. In this regard, the president of Kobayashi Pharmaceutical admitted that the decision was slow.
Another five Japanese companies issued a recall announcement.
Among the 18.5 tons of monascus raw materials produced by Kobayashi Pharmaceutical in 2023, about 16 tons of raw materials were sold to other companies for brewing and food manufacturing, except for their own health care products. At present, Kobayashi Pharmaceutical has issued a recall request to all 52 enterprises that purchased its monascus raw materials through its sales company. Affected by this, some food, brewing and other enterprises that use Xiaolin Pharmaceutical’s red yeast raw materials have also announced the recall of related products.
It is understood that the company is working hard to confirm the sales partners and recalls, and it is expected that the recall scale will be further expanded.

The reporter’s investigation found that at present, five Japanese companies have issued relevant recall announcements and announced related products. The names of the five enterprises are respectively Baojiuzao Co., Ltd., ZERO PLUS Co., Ltd., Doufu Co., Ltd. Nagoya Store, Takeya Co., Ltd. and Jiwen Food Co., Ltd.
Baojiuzao Co., Ltd.
On March 24th, Japan Baojiu Brewery Co., Ltd. announced that it had decided to recycle about 96,000 bottles of this product by itself because a wine produced by this company used red yeast raw materials provided by Kobayashi Pharmaceutical Company. At present, there are no reports of health damage related to this product of Baojiu Brewery Company.

According to the wine manufacturer, this wine has only been exported to Singapore and Taiwan, China, and has not entered the Chinese mainland market.
ZERO PLUS co., ltd
Japan’s ZERO PLUS Company issued a recall notice on March 22nd, saying that there may be unknown ingredients in the raw materials of red yeast in the thick cheese biscuits sold by its KUMITE brand. At present, the specific information of this ingredient cannot be determined. In order to prevent the safety problem from further expanding, it will be recycled independently from now on.

Doufu corporation company Nagoya branch
The recall announcement issued by the Nagoya Pastry Store of Doufu Co., Ltd. said that because its eight products contain red rice malt ingredients produced by Kobayashi Pharmaceutical, there are potential health risks, so they will take the initiative to recall these products and suspend sales.

Takeya corporation
On March 25th, Takeya, a Japanese miso production company, issued a notice that the product "Low-salt Monascus Miso" used the raw materials of Monascus produced by Kobayashi Pharmaceutical, and the sales of this product will be stopped from now on and recycled independently.

Jiwen food co., ltd
Japan’s Jiwen Food Company issued a notice on March 24th, stating that the products "domestic squid pickled products" and "squid pickled products" used the raw materials of red koji produced by Kobayashi Pharmaceutical, so they were recycled independently from now on. If the products are sent to the address designated by the company, the company will refund the payment in the future.

Xiaolin Pharmaceutical’s market value evaporated by over 4.2 billion yuan.
It is reported that Kobayashi Pharmaceutical (China) Co., Ltd. is a wholly-owned subsidiary of Kobayashi Pharmaceutical Co., Ltd.
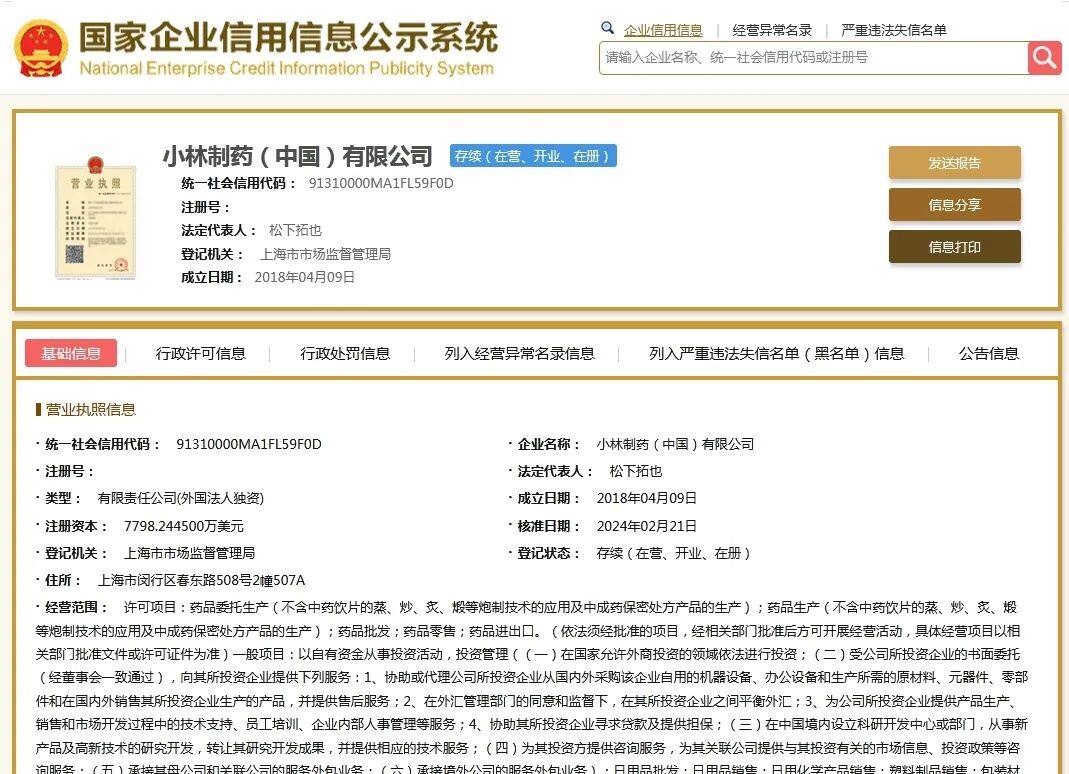
According to the national enterprise credit information publicity system, Kobayashi Pharmaceutical (China) Co., Ltd. was established in Shanghai on April 9, 2018 with a registered capital of US$ 77,982,400. The legal representative is Matsushita Tuoye, and the company is 100% owned by Kobayashi Pharmaceutical Co., Ltd.
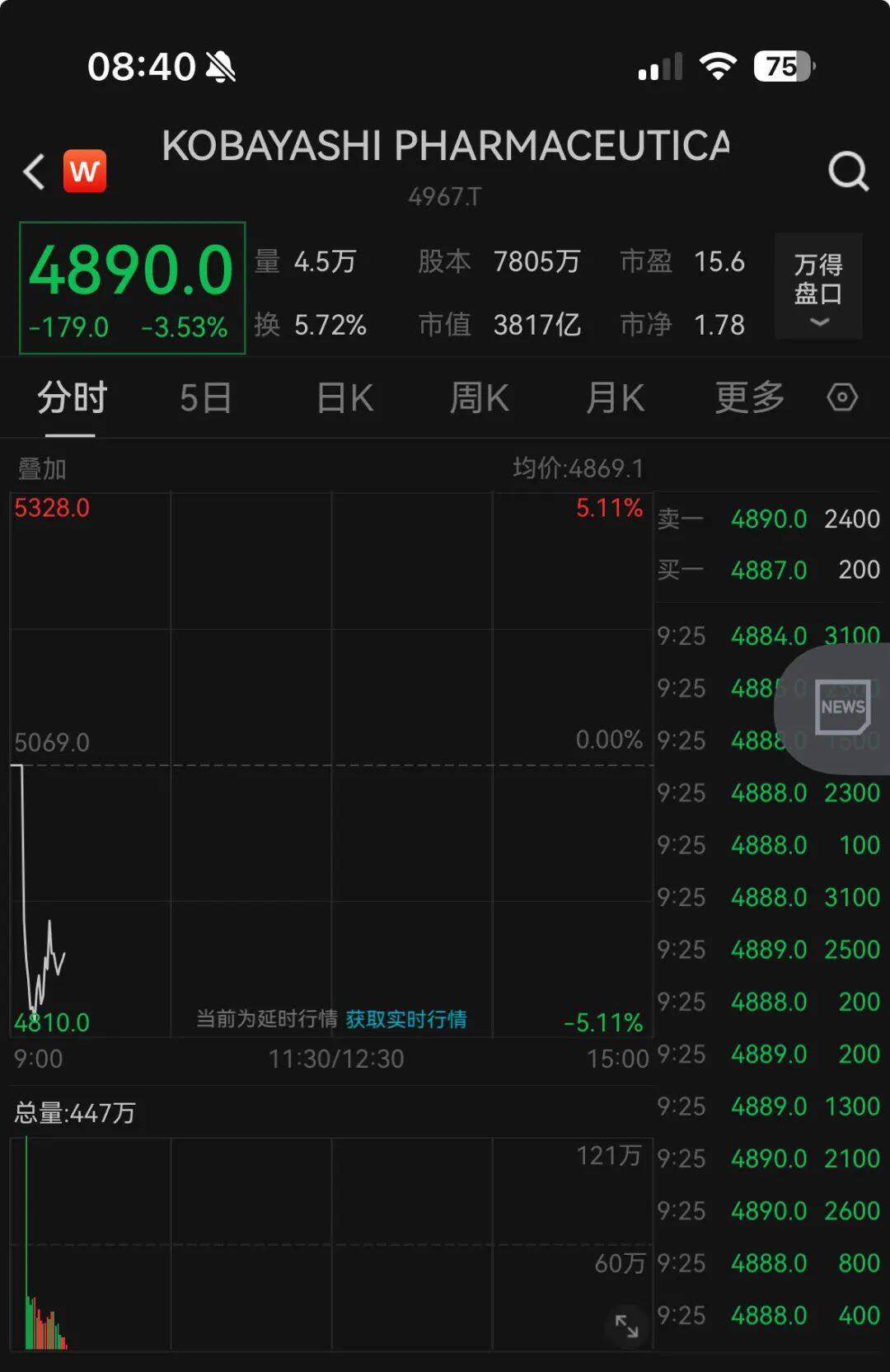
As of the morning of 27th, the share price of Kobayashi Pharmaceutical Co., Ltd. fell by 3.53%, with a total market value of 381.7 billion yen. Since the fermentation of the event, Kobayashi Pharmaceutical has fallen by nearly 20%, and its total market value has evaporated by more than 90 billion yen (about RMB 4.29 billion) compared with the closing on March 22.
Netizen: I didn’t expect health care products to be fatal.
In response to this matter, some netizens said that it was "terrible", "too scary" and "I didn’t expect health care products to be deadly", and some netizens were worried about whether other products such as warm babies and antipyretic stickers of this brand could still be used.






(Yangcheng Evening News Yangcheng School Comprehensive CCTV News, Beijing Daily, Xinhua News Agency, China News Network, Zhongxin Jingwei, Overseas Network, Beijing Youth Daily, User Comments)
Original title: Health care products are still dying? 2 deaths and 106 people have been hospitalized! Kobayashi Pharmaceutical urgently recalled, and these products should also be noted →